Client Overview
For the last 20 years Dexcom has been innovating and developing simpler and better ways for people with type 1 diabetes to measure, track and manage their diabetes. They do this through the manufacturing and distribution of their glucose monitoring systems for diabetes management.
Dexcom to date has eliminated the need for over 11 billion fingersticks and have successfully launched the world’s first real-time, integrated CGM system.
Learn how their partnership with My Language Connection, contributed to their international expansion into 52 countries, as well as supporting in getting their first EU patient into clinical trials, far faster than standard industry timelines.
MLC helped us to completely streamline our internal processes, speeding up our communications and increasing efficiency, allowing us to get our first human patient to trials, in Europe, a lot faster than we had planned through their expedited translation service.
Requirements & Challenges
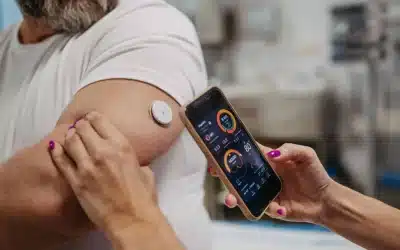
As a pioneer in Real-Time Continuous Glucose Management (CGM), Dexcom’s goal is to simplify and improve diabetes management for every possible person with diabetes. The Dexcom G6 Continuous Glucose Monitoring System sends real-time glucose readings to users smartphones or watch, meaning that with a quick glance user can see their glucose levels – all without finger-sticks or scanning.
Dexcom asked MLC to help deploy marketing and user manual documentation for a variety of territories in a bid to expedite their entry-to-market timelines. Their Diabetes technology is now available in 52 countries worldwide.
Our Approach & Solution
My Language Connection provided translation services and high-level proofreading. MLC supplied native language subject matter experts which increased the speed and accuracy in delivering translated documentation. The translated documents were marketing materials for medical devices which helped spread awareness of Dexcom’s products across Europe, the Middle East and Africa.
The services My Language Connection provided supported Dexcom through their growth, and we continue to work with Dexcom on an ongoing basis, translating technical medical documentation, marketing materials and even social media management in multiple languages.
At MLC we are experts in providing companies within the healthcare & medical industry with expert language services. Some of our expertise include biotech translations, pharmaceutical translation, medical device translations as well as providing a wide range of medical translation services. Our partnership approach and commitment to quality mean we can help our clients improve outcomes and achieve their strategic goals.
Our On-Going Support
We are proud to have supported Dexcom for over 7 years and counting, by translating their content across the product life cycle – from study documents to gather clinical data, through product information and labelling for product registration, to promotional materials for brand launch and ongoing marketing campaigns and expansion.
We have contributed to the success of their product launches relating to G5, G6, G7, Dexcom One and Dexcom One +. Furthermore, with Dexcom’s feedback we developed the MLC Translation Management System. Our technology offers Dexcom a bespoke virtual Project Management workspace for their team to track their budgets and projects in real time; for In Country Reviewers to make changes to projects that are automatically logged into their glossaries; and ultimately get their medical devices to market 50% + faster.
Learn more from Dexcom and their experience with the MLC TMS below.
The Crucial Role of Translation in Medical Device Labelling
The Crucial Role of Translation in Medical Device Labelling As medical device companies expand globally, the need for precise and effective translations becomes critical to ensure safety, regulatory compliance, and market success. This exploration delves into the intricacies of medical device labelling translations, highlighting key considerations and best practices for achieving high-quality outcomes. Ensuring that health information is...
My Language Connection’s Journey to the Glasgow Business Awards
We have been shortlisted! We are thrilled to announce that My Language Connection has been shortlisted for the prestigious Best Performing Small to Medium-Sized Business of the Year at the upcoming Glasgow Business Awards. This recognition is a testament to the dedication and hard work of our entire team in providing exceptional service across Scotland. To our valued clients, we extend our heartfelt gratitude for your unwavering support and...
Quality Control in Medical Device Translation Services?
What is Quality Control in Medical Device Translation Services? In the global healthcare industry, precise communication is critical. Medical devices, which range from simple instruments to complex machinery, often come with detailed instructions, specifications, and regulatory requirements. When these documents need translation, ensuring accuracy and compliance is paramount. This is where quality control in medical device translation services...
Which factors are important in selecting a translation service provider?
By What Criteria Should You Select a Translation Service Provider? The need for accurate and effective translation services has never been more crucial. Whether you're a business looking to expand into new markets, a researcher needing to translate scientific documents, or an individual requiring personal translations, selecting the right translation service provider is paramount. But with so many options available, how do you make the...
Honouring Excellence: My Language Connection Shortlisted for ATC Language Industry Awards 2024
We are thrilled to announce that My Language Connection has been shortlisted as a finalist for The New Member of the Year 2024 Award.
What are the key objectives of medical device translation?
What are the Key Objectives of Medical Device Translation? In the rapidly evolving global healthcare landscape, the importance of precise and effective medical device translation cannot be overstated. As medical devices continue to play a critical role in patient care, ensuring that all related documentation and information are accurately translated is essential for several reasons. These translations must adhere to stringent regulatory...